
The Science of Convenient

THE SCIENCE OF CONVENIENT
Alfalfa-based top-dress pellet for the treatment of equine protozoal myeloencephalitis (EPM) in horses.
- Safe, easy and effective, Protazil® (1.56% diclazuril) antiprotozoal pellets start working fast against a disease where time matters
- First FDA-approved alfalfa-based, pelleted formulation containing diclazuril
- Rapid absorption and no loading dose required1
- Makes accurate dosing and administration easier and safer for horse owners
- Well accepted by horses and consumed with no mess or fuss
- Administer as a daily top-dress at 1 mg/kg for 28 days
- Important Safety Information
Use of Protazil (1.56% dicazuril) is contraindicated in horses with known hypersensitivity to diclazuril. Safe use in horses used for breeding purposes, during pregnancy, or in lactating mares has not been evaluated. The safety of Protazil (1.56% dicazuril) with concomitant therapies in horses has not been evaluated. For use in horses only. Do not use in horses intended for human consumption. Not for human use. Keep out of reach of children.
INDICATIONS
PROTAZIL (1.56% diclazuril) Antiprotozoal Pellets are indicated for the treatment of equine protozoal myeloencephalitis (EPM) caused by Sarcocystis neurona in horses.
ADMINISTRATION
Dose
PROTAZIL (1.56% diclazuril) is administered as a top dress in the horse’s daily grain ration at a rate of 1 mg diclazuril per kg (0.45 mg diclazuril/lb) of body weight for 28 days. The quantity of PROTAZIL necessary to deliver this dose is 64 mg pellets per kg (29 mg pellets/lb) of body weight.
Administration
To achieve this dose, weigh the horse (or use a weight tape). Scoop up PROTAZIL to the level (cup mark) corresponding to the dose for the horse’s body weight using the following chart:
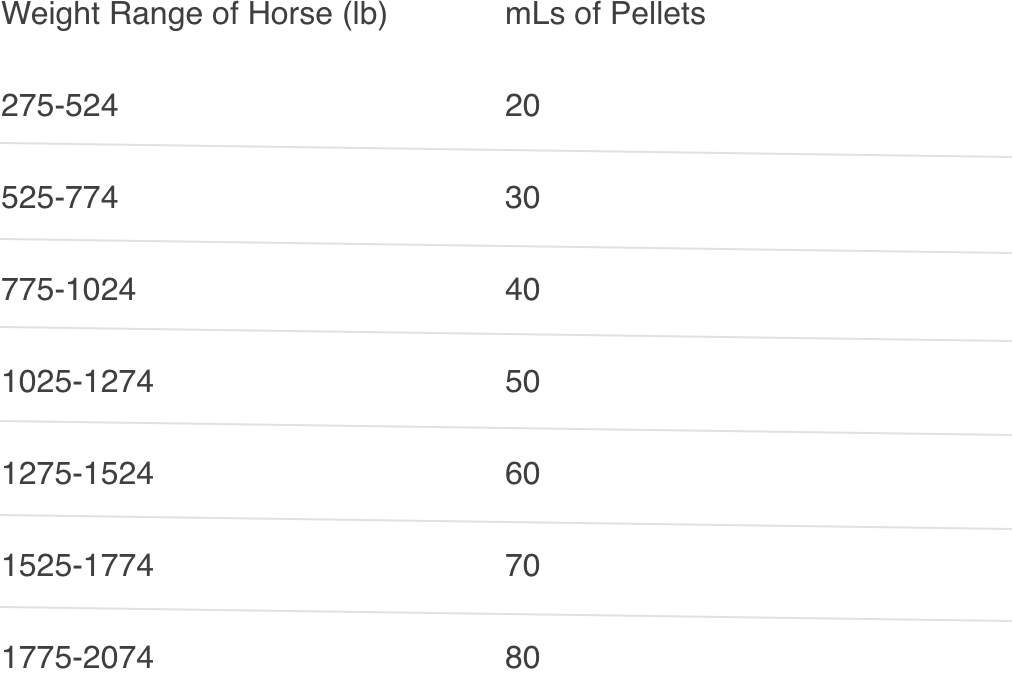
One 2.4-lb bucket of PROTAZIL will treat one 1274-lb horse for 28 days.
Animal Safety
In a second target animal safety study, PROTAZIL (1.56% diclazuril) Antiprotozoal Pellets were administered to 24 horses (12 males and 12 females, ranging from 2 to 8 years of age). Three groups of 4 horses/sex/group received 0, 1, or 5 mg diclazuril/kg body weight/day for 42 days as a topdress on the grain ration of the horse. The variables measured during the study included physical examinations, body weights, food and water consumption, hematology, and serum chemistry. There were no test article-related findings seen during the study.
Contraindications
Use of PROTAZIL (1.56% diclazuril) Antiprotozoal Pellets is contraindicated in horses with known hypersensitivity to diclazuril.
Warnings
For use in horses only. Do not use in horses intended for human consumption. Not for human use. Keep out of reach of children.
Precautions
The safe use of PROTAZIL (1.56% diclazuril) Antiprotozoal Pellets in horses used for breeding purposes, during pregnancy, or in lactating mares has not been evaluated. The safety of PROTAZIL (1.56% diclazuril) Antiprotozoal Pellets with concomitant therapies in horses has not been evaluated.
RESOURCES
Keep your clinic up-to-date on the Merck Animal Health line of equine products.


