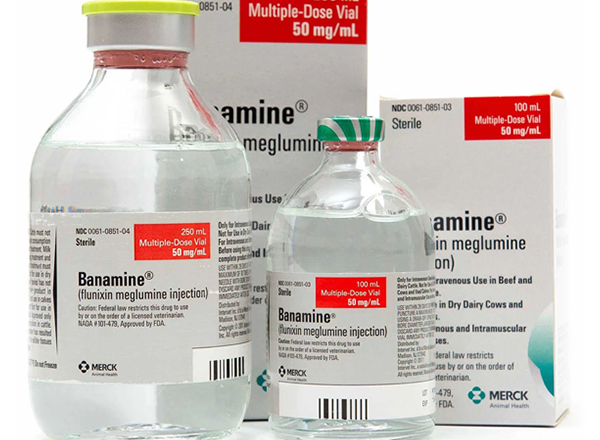
BANAMINE® (flunixin meglumine injection) for Cattle
Banamine brand of flunixin meglumine is the pioneer injectable non steroidal anti-inflammatory drug approved for cattle and horses in the United States.
(flunixin meglumine)
Related Products
Banamine® Transdermal
(flunixin transdermal solution)
Overview
Pharmaceutical Name
Flunixin meglumine
Overview
Banamine brand of flunixin meglumine is the pioneer injectable nonsteroidal anti-inflammatory drug approved for cattle and horses in the United States.
Banamine reduces the fever and lung inflammation that typically accompany bovine respiratory disease (BRD). With Banamine as part of a BRD treatment program, cattle feel better fast. Cattle pulled for BRD and treated with Banamine (flunixin meglumine) in addition to an antibiotic visited the feed bunk more frequently (P < 0.10), spent more time at the feed bunk (P < 0.05), and had significantly reduced rectal temperature during a 12-hour period post-treatment than cattle treated with an antibiotic alone. They also have reduced lung inflammation and fewer lung lesions than cattle treated with conventional therapies.1
Each milliliter of BANAMINE contains flunixin meglumine equivalent to 50mg flunixin, 0.1mg edetate disodium, 2.5mg sodium formaldehyde sulfoxylate, 4.0mg diethanolamine, 207.2mg propylene glycol; 5.0mg phenol as preservative, hydrochloric acid, water for injection qs.
Safety
Horse: A three-fold intramuscular dose of 1.5mg/lb of body weight daily for 10 consecutive days was safe. No changes were observed in hematology, serum chemistry, or urinalysis values. Intravenous dosages of 0.5mg/lb daily for 15 days; 1.5mg/lb daily for 10 days; and 2.5mg/lb daily for five days produced no changes in blood or urine parameters. No injection site irritation was observed following intramuscular injection of the 0.5mg/lb recommended dose. Some irritation was observed following a three-fold dose administered intramuscularly.
Cattle: No flunixin-related changes (adverse reactions) were noted in cattle administered a 1X (2.2mg/kg; 1.0mg/lb) dose for nine days (three times the maximum clinical duration). Minimal toxicity manifested itself at moderately elevated doses (3X and 5X) when flunixin was administered daily for nine days, with occasional findings of blood in the feces and/or urine. Discontinue use if hematuria or fecal blood are observed.
Indications
Horse: BANAMINE is recommended for the alleviation of inflammation and pain associated with musculoskeletal disorders in the horse. It is also recommended for the alleviation of visceral pain associated with colic in the horse.
Cattle: BANAMINE is indicated for the control of pyrexia associated with bovine respiratory disease, endotoxemia and acute bovine mastitis. BANAMINE is also indicated for the control of inflammation in endotoxemia.
Administration and Dosage
Horse: The recommended dose for musculoskeletal disorders is 0.5mg per pound (1 mL/100 lbs) of body weight once daily. Treatment may be given by intravenous or intramuscular injection and repeated for up to five days. Studies show onset of activity within two hours. Peak response occurs between 12 and 16 hours and duration of activity is 24 to 36 hours.
The recommended dose for the alleviation of pain associated with equine colic is 0.5mg per pound of body weight. Intravenous administration is recommended for prompt relief. Clinical studies show pain is alleviated in less than 15 minutes in many cases. Treatment may be repeated when signs of colic recur. During clinical studies approximately 10% of the horses required one or two additional treatments. The cause of colic should be determined and treated with concomitant therapy.
Cattle: The recommended dose for cattle for control of pyrexia associated with bovine respiratory disease and endotoxemia and control of inflammation in endotoxemia is 1.1 to 2.2mg/kg (0.5 to 1mg/lb; 1 to 2 mL per 100 lbs) of body weight given by slow intravenous administration either once a day as a single dose or divided into two doses administered at 12-hour intervals for up to three days. The total daily dose should not exceed 2.2mg/kg (1.0mg/lb) of body weight. Avoid rapid intravenous administration of the drug.
The recommended dose for acute bovine mastitis is 2.2mg/kg (1mg/lb: 2 mL per 100 lbs) of body weight given once by intravenous administration.
Precautions
As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal and renal toxicity. Sensitivity to drug-associated adverse effects varies with the individual patient. Patients at greatest risk for renal toxicity are those that are dehydrated, on concomitant diuretic therapy, or those with renal, cardiovascular, and/or hepatic dysfunction.
Since many NSAID’s possess the potential to induce gastrointestinal ulceration, concomitant use of BANAMINE with other anti-inflammatory drugs, such as other NSAIDs and corticosteroids, should be avoided or closely monitored.
Horse: The effect of BANAMINE on pregnancy has not been determined. Studies to determine activity of BANAMINE when administered concomitantly with other drugs have not been conducted. Drug compatibility should be monitored closely in patients requiring adjunctive therapy.
Cattle: Do not use in bulls intended for breeding, as reproductive effects of BANAMINE in these classes of cattle have not been investigated. NSAIDs are known to have potential effects on both parturition and the estrous cycle. There may be a delay in the onset of estrus if flunixin is administered during the prostaglandin phase of the estrous cycle. NSAIDs are known to have the potential to delay parturition through a tocolytic effect. Do not exceed the recommended dose.
Supplied
BANAMINE, 50mg/mL is available in 100-mL (NDC 0061-0851-03) and 250-mL (NDC 0061-0851-04) multi-dose vials. Store between 2° and 30° C (36° and 86° F).
Fair Balance
RESIDUE WARNINGS: Cattle must not be slaughtered for human consumption within four days of the last treatment. Milk that has been taken during treatment and for 36 hours after the last treatment must not be used for food. Not for use in dry dairy cows. A withdrawal period has not been established for this product in preruminating calves. Do not use in calves to be processed for veal. Not for use in horses intended for food. Approved only for intravenous administration in cattle. Intramuscular administration has resulted in violative residues in the edible tissues of cattle sent to slaughter.
Contact Information
US only: Merck Animal Health livestocktechsrvc@merck-animal-health.com 1-800-211-3573
For additional information, please see the product label.
References:
- Techincal Bulletin SPAH-BA-58.
Technical Information
Flunixin meglumine is a potent, non-narcotic, nonsteroidal, analgesic agent with anti-inflammatory and antipyretic activity. It is significantly more potent than pentazocine, meperidine, and codeine as an analgesic in the rat yeast paw test.
Horse: Flunixin is four times as potent on amg-per-mg basis as phenylbutazone as measured by the reduction in lameness and swelling in the horse. Plasma half-life in horse serum is 1.6 hours following a single dose of 1.1mg/kg. Measurable amounts are detectable in horse plasma at eight hours post injection.
Cattle: Flunixin meglumine is a weak acid (pKa=5.82) which exhibits a high degree of plasma protein binding (approximately 99%). However, free (unbound) drug appears to readily partition into body tissues (Vss predictions range from 297 to 782 mL/kg. Total body water is approximately equal to 570 mL/kg). In cattle, elimination occurs primarily through biliary excretion. This may, at least in part, explain the presence of multiple peaks in the blood concentration/time profile following IV administration.
In healthy cattle, total body clearance has been reported to range from 90 to 151 mL/kg/hr. These studies also report a large discrepancy between the volume of distribution at steady state (Vss) and the volume of distribution associated with the terminal elimination phase (Vβ). This discrepancy appears to be attributable to extended drug elimination from a deep compartment. The terminal half-life has been shown to vary from 3.14 to 8.12 hours.
Flunixin persists in inflammatory tissues and is associated with anti-inflammatory properties which extend well beyond the period associated with detectable plasma drug concentrations. These observations account for the counterclockwise hysteresis associated with flunixin’s pharmacokinetic/pharmacodynamic relationships. Therefore, prediction of drug concentrations based upon the estimated plasma terminal elimination half-life will likely under-estimate both the duration of drug action and the concentration of drug remaining at the site of activity.
For additional information, please see the product label.
When Does It Make Sense to Treat for BRD? ASAP! (192kb)Bacterial populations can double every 30 minutes. With BRD, fast-acting treatment is critical. Cattle need all the help you can give.
Cattle Lungs are Naturally Prone to Infection. (188kb)The Immune System’s Reaction Can Actually Cause Damage. What to do? – Give cattle both a non-steroidal anti-inflammatory and an antibiotic for Lung Protection Therapy (LPT). – Administer Lung Protection Therapy (LPT) early to save lives and protect performance and profit.
Save Cattle and Profits with Lung Protection Therapy (Feedlot) (1738kb)
Save Cattle and Profits with Lung Protection Therapy (Dairy) (1769kb)
Save Cattle and Profits with Lung Protection Therapy (Cow Calf) (1755kb)