Banamine®
Transdermal
(flunixin transdermal solution)
CONTROL PAIN ASSOCIATED
WITH FOOT ROT.
Looking for Banamine Injectable Solution (flunixin meglumine)?
Banamine Transdermal was determined to be safe and efficacious in controlling pain associated with foot rot.
TRIAL SETUP
- Field studies in two states with 30 steers weighing between 717 and 1,085 pounds.
- Animals experimentally induced with foot rot infection in the right front limb.
- After 48 hours, animals given either Banamine Transdermal (3 mL per 100 pounds) or placebo. No antimicrobials were administered.
- Considered a treatment success if the lameness score decreased by more or equal to one point.
RESULTS
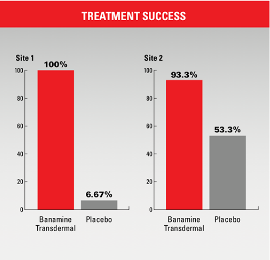
- At Site 1, lameness score treatment success was 100%, compared to 6.67% in the placebo group. Mean change in maximum total force and mean change in contact area were significantly different (p=0.0001) and higher in the flunixin transdermal solution-treated group.
- At Site 2, lameness treatment success was 93.3%, compared to 53.3% for the placebo group. Mean change in maximum total force and mean change in contact area were significantly different (p=0.0002 and p<0.0001, respectively) and higher in the flunixin transdermal solution-treated group.
The marked increases in both maximum total force and total contact area at both study sites were clinically interpreted as animals able to plant their affected foot in a more normal fashion and bear more weight on the affected limb, supporting clinical improvement in lameness as a result of less pain.
An innovation in Pain Control
IMPORTANT SAFETY INFORMATION: NOT FOR HUMAN USE.
KEEP OUT OF REACH OF CHILDREN. Only for topical use in beef and dairy cattle. Do not use Banamine Transdermal pour-on within 48 hours of expected parturition. Do not use in animals showing hypersensitivity to flunixin meglumine. Cattle must not be slaughtered for human consumption within 8 days of the last treatment. Not for use in female dairy cattle 20 months of age or older, including dry dairy cows; use in these cattle may cause drug residues in milk and/or in calves born to these cows or heifers. Not for use in suckling beef calves, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves. Not for use in dairy or beef bulls intended for breeding because reproductive safety has not been evaluated.