Banamine®
Transdermal
(flunixin transdermal solution)
BRD KEEPING THEM OFF FEED?
IT’S TIME TO CLOSE THE GAP
Looking for Banamine Injectable Solution (flunixin meglumine)?
Banamine Transdermal was determined to be safe and efficacious in controlling fever associated with BRD.
TRIAL SETUP
- Field studies in four states with 251 animals diagnosed with BRD.
- Rectal temperatures ranged from 104.5 to 107.8°F.
- Animals received either 3 mL per 100 pounds of Banamine Transdermal or a placebo. No antimicrobials were administered.
- Six hours after treatment, temperatures were measured again.
- Considered a treatment success if rectal temperature was reduced by ≥2°F.
RESULTS
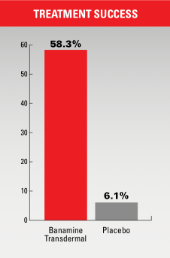
- Treatment success was 58.3% for animals receiving Banamine Transdermal compared with 6.1% for the placebo group (p<0.001).
Reduce BRD fever and get ’em back to the bunk
IMPORTANT SAFETY INFORMATION: NOT FOR HUMAN USE.
KEEP OUT OF REACH OF CHILDREN. Only for topical use in beef and dairy cattle. Do not use Banamine Transdermal pour-on within 48 hours of expected parturition. Do not use in animals showing hypersensitivity to flunixin meglumine. Cattle must not be slaughtered for human consumption within 8 days of the last treatment. Not for use in female dairy cattle 20 months of age or older, including dry dairy cows; use in these cattle may cause drug residues in milk and/or in calves born to these cows or heifers. Not for use in suckling beef calves, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves. Not for use in dairy or beef bulls intended for breeding because reproductive safety has not been evaluated.